|
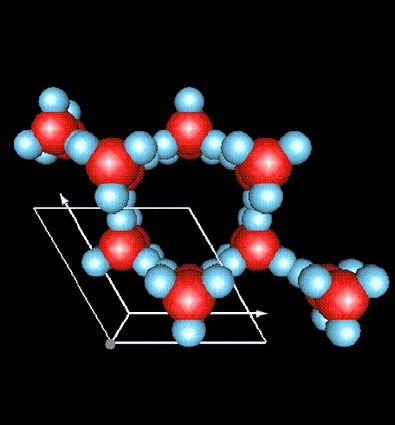
"The Reason for the Seasons:" - Snowflake Shapes
The picture above was created at the Institut Laue-Langevin an international research centre and world leader in neutron science and technology. It is based in Grenoble in the South-East of France. It shows an ice crystal. The crystal is made of many molecules of water (H O). The atoms are shown by different colors. The darker atoms are oxygen. The lighter atoms are hydrogen. The hydrogen atoms are attached at an angle near 120 degrees.
The hydrogen atoms are attracted to each other and form hexagonal rings in all directions. As ice crystals or snowflakes grow, they expand by attaching new water molecules to each other. Looking at them with a hand lens or microscope tells us about how they join together. The angles are always the same so the designs always have six sides. Whether ice crystals or snowflakes, observing the shape under "atomic microscopes" reveals a shape that is always hexagonal.
If the angle had been different, the shape would have been different. Salt crystals (NaCl) are made of two elements, sodium (Na) and chlorine (Cl) which join at 90 degree angles. Under a hand lens or microscope, the crystals of salt appear as little dice or cubes. The shape of the crystal is determined by the angle of chemical bonding (joining together).
What does "ice" have to do with "ice cream"?
Below is a typical triglyceride butterfat molecule from which ice cream is made. Ice cream is formed when many tiny ice crystals form between the "arms" of the triglyceride butterfat molecule.
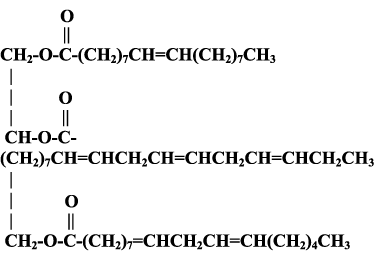
Typical molecule of butterfat, a triglyceride, found in ice cream.
Extensions: Chemistry, Crystals and Calories
- Look at the drawing of the butterfat molecule. The letters stand for chemical elements, joined together in long chains. You can make a "MODEL" of the molecules with gum drops and toothpicks
- You can make up a code... which element (gumdrop) is which color:
The elements are:
Carbon (C) Color
Oxygen (O) Color
Hydrogen (H) Color
- Build the molecule with groups assembling a part of a chain. Connect them with toothpicks (chemical bonds... the glue that holds elements together in molecules). . The symbols "=" or "||" mean use two toothpicks. These are called double bonds in chemistry. Then lay them out and connect the whole butterfat molecule on the floor or table.
- At the same time, make lots of water molecules (H2O- Oxygen in middle, Hydrogens on each side like a boomerang) and oxygen molecules (O2). Lay the water molecules between the long chains of the butterfat. Now "freeze" them by connecting three boomerang shaped water molecules together in a hexagon shape, touching the hydrogen atoms together.
- Why does ice cream make people gain weight? After you eat ice cream, the only way to get rid of it is to "burn" it out of your body. That involves the same idea as burning a match... fuel and oxygen... except this burning is flameless. The ice cream is the fuel and the air you breathe gives you oxygen.
- "Burn" the ice cream by using the oxygen molecules you made. Oxygen breaks ice cream apart by attacking and breaking the toothpicks and carrying away the Hydrogen and Carbon. Here is the formula:
C + O2 makes one CO2 (carbon dioxide you breath out)
H + H+O makes one H2O (water) which you breath out (cold morning breath?)
- How many oxygens does it take to carry away the butterfat molecule? This is why "aerobics" is a good idea for weight loss... makes you fill your body with lots of oxygen to "burn" the butterfat, releasing "heat" measured in calories (a way of measuring energy content).
The Thermodynamics and Chemistry of Ice Cream
(Where is the heat going and what happens to ice cream after you eat it?) What is going on in the bags?
- The inside of the ice is very cold, -10° to -20° F. But when you hold an ice cube, the exterior, in contact with air and your hand is +32° F, cold water. Clean, pure water cannot be a liquid below +32° F. It becomes ice.
- Salt Mysteries- The mixture of salt and water can be liquid below +32° F. It can be a liquid down to -20° F. So adding salt does not "melt ice". It makes a mixture of water and salt that has a low temperature... "Salt gives water permission to freeze at a lower temperature".
- The very cold salt water surrounds the baggie with the milk (which is 30% water) and "steals" heat from the milk. The temperature of the milk becomes so cold that the water in the milk begins to form tiny ice crystals. The butterfat does not form crystals. The shaking keeps the milk from forming one big ice cube.
- What is a comet - ice cube or ice cream? Deep Impact will help us find out. The data from Deep Impact will tell us a little about how the comet formed... blob of water or snowball of crystals that came together.
Art Hammon, Pre-college Education Specialist, JPL
ahammon@jpl.nasa.gov
|
